Notification procedure
http://echa.europa.eu/regulations/biocidal-products-regulation/approval-of-active-substances/existing-active-substance/notification-procedure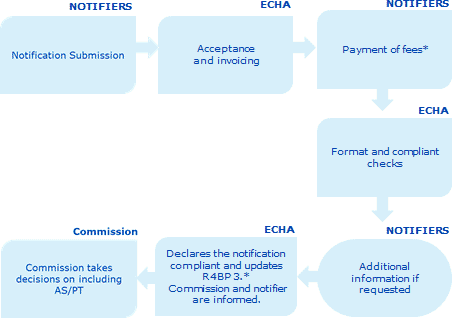
The notification process to take over the role of participant or to include an active substance/product-type in the Review Programme has the following steps:
A notifier (prospective participant) submits a notification in a IUCLID file through R4BP 3 containing the information listed in Annex I of the Review Programme Regulation

ECHA accepts the application and informs the notifier who has 30 days to pay the applicable fees.
The notifier pays the related fees to ECHA within 30 days. In case payment is not made within the deadline, the notification will be rejected.
Upon receipt of the fee payment, ECHA verifies within 30 days that the notification has been submitted in the correct format and contains the data requirements listed in Annex I of Review Programme Regulation (EU) N0 1062/2014.

If the notification is not compliant, ECHA will grant the notifier 30 days to correct or complete the notification.
ECHA declares the notification as compliant or it rejects the notification if it considers it not to be compliant.*

When a notification is considered compliant, the European Commission takes the decision to include the substance/product-type combination in the Review Programme.
Actors
The main actors in the notification process are:
- Notifiers
The notifiers are the prospective participants. The notifiers are responsible for providing all necessary information in their notifications. They should pay attention to the various deadlines throughout the process. - ECHA
ECHA performs the format and compliance check of the notifications and declares it compliant or not. - European Commission
The European Commission takes into consideration the declaration by ECHA on the compliance of the notification and, if relevant, includes the substance/PT combination in the Review Programme.
For substance/product-type combinations which are no longer supported, in the absence of any compliant notification by the applicable deadline, or if the notification is rejected, or where the substance identity is not fully covered, the substance/product-type will be the subject of a Commission non-approval decision in accordance with Article 89(1) of the BPR.
Related links
- Guidance on information requirements [PDF]
- Product-types
- How to submit a notification in the Review Program (Role of participant or for inclusion of a new Active substance)
http://echa.europa.eu/publications
Keine Kommentare:
Kommentar veröffentlichen