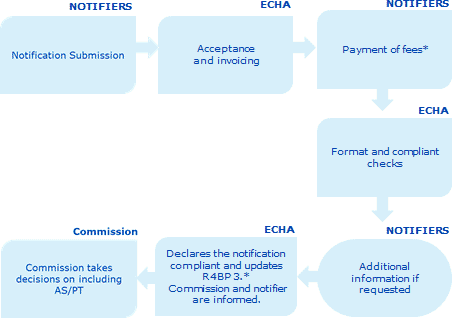
According to the Directive 98/8/EC until the inclusion of the active substance (transitional period) the placing on the market of the biocidal products are regulated by the national regulations in force.
To see the details of the Hungarian regulations in force please click below on the product type of your interest.
Zulassungen, Anträge, Adressen usw. in UNGARN
Product type 1: Human hygiene biocidal products
Products in this group are biocidal products used for human hygiene purposes.
Products in this group are biocidal products used for human hygiene purposes.
Product type 2: Private area and public health area disinfectants and other biocidal productsa.) Private area and public health area and disinfectants and other biocidal products
b.) Products used for the disinfection of swimming pools, aquariums, bathing and other waters
b.) Products used for the disinfection of swimming pools, aquariums, bathing and other waters
Products used for the disinfection of air, surfaces, materials, equipment and furniture which are not used for direct food or feed contact in private, public and industrial areas, including hospitals, as well as products used as algaecides.
Usage areas include, inter alia, swimming pools, aquariums, bathing and other waters; air-conditioning systems; walls and floors in health and other institutions; chemical toilets, waste water, hospital waste, soil or other substrates (in playgrounds).
Usage areas include, inter alia, swimming pools, aquariums, bathing and other waters; air-conditioning systems; walls and floors in health and other institutions; chemical toilets, waste water, hospital waste, soil or other substrates (in playgrounds).
Product type 3: Veterinary hygiene biocidal products a.) Surface or airspace disinfectant products for veterinary purposes
b.) Veterinary hygiene biocidal products
b.) Veterinary hygiene biocidal products
Products in this group are biocidal products used for veterinary hygiene purposes including products used in areas in which animals are housed, kept or transported.
Product type 4: Food and feed area disinfectants
Products used for the disinfection of equipment, containers, consumption utensils, surfaces or pipework associated with the production, transport, storage or consumption of food, feed or drink (including drinking water) for humans and animals.
Products used for the disinfection of equipment, containers, consumption utensils, surfaces or pipework associated with the production, transport, storage or consumption of food, feed or drink (including drinking water) for humans and animals.
Product type 5: Drinking water disinfectants
Products used for the disinfection of drinking water (for both humans and animals).
Products used for the disinfection of drinking water (for both humans and animals).
Product type 6: In-can preservatives
Products used for the preservation of manufactured products, other than foodstuffs or feedingstuffs, in containers by the control of microbial deterioration to ensure their shelf life.
Products used for the preservation of manufactured products, other than foodstuffs or feedingstuffs, in containers by the control of microbial deterioration to ensure their shelf life.
Product type 7: Film preservatives
Products used for the preservation of films or coatings by the control of microbial deterioration in order to protect the initial properties of the surface of materials or objects such as paints, plastics, sealants, wall adhesives, binders, papers, art works.
Products used for the preservation of films or coatings by the control of microbial deterioration in order to protect the initial properties of the surface of materials or objects such as paints, plastics, sealants, wall adhesives, binders, papers, art works.
Product type 8: Wood preservatives
Products used for the preservation of wood, from and including the saw-mill stage, or wood products by the control of wood-destroying or wood-disfiguring organisms.
This product type includes both preventive and curative products.
Products used for the preservation of wood, from and including the saw-mill stage, or wood products by the control of wood-destroying or wood-disfiguring organisms.
This product type includes both preventive and curative products.
Product type 9: Fibre, leather, rubber and polymerised materials preservatives
Products used for the preservation of fibrous or polymerised materials, such as leather, rubber or paper or textile products and rubber by the control of microbiological deterioration.
Products used for the preservation of fibrous or polymerised materials, such as leather, rubber or paper or textile products and rubber by the control of microbiological deterioration.
Product type 10: Masonry preservatives
Products used for preservation and remedial treatment of masonry or other construction materials other than wood by the control of microbiological and algal attack.
Products used for preservation and remedial treatment of masonry or other construction materials other than wood by the control of microbiological and algal attack.
Product type 11: Preservatives for liquid-cooling and processing systems
Products used for the preservation of water or other liquids used in cooling and processing systems by the control of harmful organisms such as microbes, algae and mussels.
Products used for the preservation of drinking water are not included in this product type.
Products used for the preservation of water or other liquids used in cooling and processing systems by the control of harmful organisms such as microbes, algae and mussels.
Products used for the preservation of drinking water are not included in this product type.
Product type 12: Slimicides
Products used for the prevention or control of slime growth on materials, equipment and structures, used in industrial processes, e.g. on wood and paper pulp, porous sand strata in oil extraction.
Products used for the prevention or control of slime growth on materials, equipment and structures, used in industrial processes, e.g. on wood and paper pulp, porous sand strata in oil extraction.
Product type 13: Metalworking-fluid preservatives
Products used for the preservation of metalworking fluids by the control of microbial deterioration.
Products used for the preservation of metalworking fluids by the control of microbial deterioration.
Product type 14: Rodenticides a.) Products used against health pests
b.) Products used against non-health pests
Products used for the control of mice, rats or other rodents.
b.) Products used against non-health pests
Products used for the control of mice, rats or other rodents.
Product type 15: Avicides
Products used for the control of birds.
Products used for the control of birds.
Product type 16: Molluscicides
Products used for the control of molluscs.
Products used for the control of molluscs.
Product type 17: Piscicides
Products used for the control of fish; these products exclude products for the treatment of fish diseases.
Products used for the control of fish; these products exclude products for the treatment of fish diseases.
Product type 18: Insecticides, acaricides and products to control other arthropods a.) Products used against health pests
b.) Products used for animal purposes
c.) Products used for other purposes
Products used for the control of arthropods (e.g. insects, arachnids and crustaceans).
b.) Products used for animal purposes
c.) Products used for other purposes
Products used for the control of arthropods (e.g. insects, arachnids and crustaceans).
Product type 19: Repellents and attractants a.) Products used against health pests
b.) Products used for animal purposes
c.) Products used for other purposes
Products used to control harmful organisms (invertebrates such as fleas, vertebrates such as birds), by repelling or attracting, including those that are used for human or veterinary hygiene either directly or indirectly.
b.) Products used for animal purposes
c.) Products used for other purposes
Products used to control harmful organisms (invertebrates such as fleas, vertebrates such as birds), by repelling or attracting, including those that are used for human or veterinary hygiene either directly or indirectly.
Product type 20: Preservatives for food or feedstocks
Products used for the preservation of food or feedstocks by the control of harmful organisms.
Products used for the preservation of food or feedstocks by the control of harmful organisms.
Product type 21: Antifouling products
Products used to control the growth and settlement of fouling organisms (microbes and higher forms of plant or animal species) on vessels, aquaculture equipment or other structures used in water.
Products used to control the growth and settlement of fouling organisms (microbes and higher forms of plant or animal species) on vessels, aquaculture equipment or other structures used in water.
Product type 22: Embalming and taxidermist fluids
Products used for the disinfection and preservation of human or animal corpses, or parts thereof.
Products used for the disinfection and preservation of human or animal corpses, or parts thereof.
Product type 23: Control of other vertebrates
Products used for the control of vermin.
Products used for the control of vermin.